
Sunnybrook Research Institute senior scientist Dr. Isabelle Aubert and her PhD student, Kristiana Xhima, led the first study using focused ultrasound to deliver a molecule to the brain to revive the function of neurons vital to learning and memory in mice with Alzheimer’s disease.
Breakthrough targets restoring the function of neurons vital to learning and memory
While billions of dollars have been poured into finding a cure for Alzheimer’s disease, progress has been frustratingly slow. Approved treatments for the disorder reduce symptoms, but there is nothing that can stop its destructive course.
Against this backdrop, neuroscientists at Sunnybrook Research Institute (SRI) are developing strategies to counter brain degeneration in Alzheimer’s disease. One approach, led by SRI senior scientist Dr. Isabelle Aubert, and her PhD student, Kristiana Xhima, is showing promise. They completed the first preclinical study using focused ultrasound to deliver a compound that rescues the function of brain cells that are impaired in Alzheimer’s disease.

The researchers targeted a receptor on these neurons that caused the neurons to release a chemical messenger, a sign of restored function. The pivotal study was published in Science Advances on Jan. 22, 2020.
Their method restores communication between cholinergic neurons, brain cells that are essential for learning and memory. This communication is short-circuited in Alzheimer’s to devastating effect. In a healthy brain, neurons connect at synapses, where chemical messengers called neurotransmitters carry information from one cell to another. For example, cholinergic neurons transmit acetylcholine. Alzheimer’s disease impedes this vital transmission. Toxic proteins that accumulate in the brain, amyloid and tau, are thought to lead to the loss of neurons; as brain cells die, there are fewer neurotransmitters, like acetylcholine, to carry messages between cells.
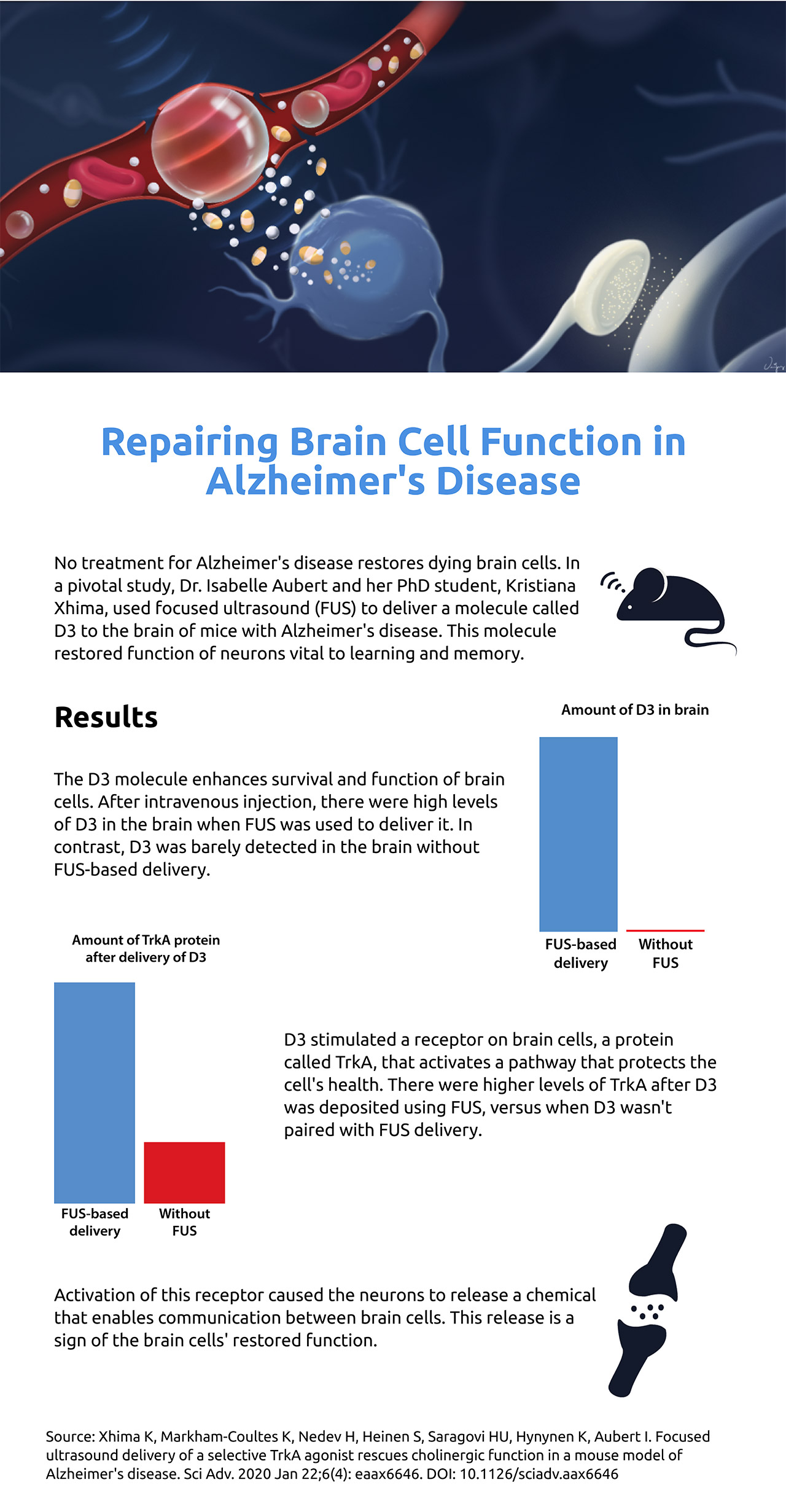
Aubert and Xhima used focused ultrasound to deliver noninvasively a molecule called D3 across the blood-brain barrier, precisely where cholinergic neurons reside, into the brains of mice with Alzheimer’s disease. They worked with Dr. Kullervo Hynynen, a pioneer of focused ultrasound who was the first to show that the technology could locally and temporarily open the blood-brain barrier under MRI guidance. The molecule mimics a protein called nerve growth factor (NGF), which is made in the brain and is important to the health of cholinergic neurons. Early in Alzheimer’s disease, there are changes in how these neurons access and use NGF.
In trying to revive ailing neurons, researchers previously have delivered NGF-based therapies to the brain, but have encountered roadblocks. One is the blood-brain barrier, which protects the brain from circulating toxins, but also blocks drugs and other therapies. Because of this, NGF has been given via holes made in the skull, coupled with devices that penetrate the brain tissue—a risky procedure, particularly for frail patients.
In contrast, focused ultrasound is noninvasive. It uses microbubbles and an MRI contrast agent that are infused intravenously. When the bubbles reach the target area, as visualized by MRI—in this case, the basal forebrain, where cholinergic neurons are found—acoustic energy is applied, causing the bubbles to jostle the tight junctions between cells that make up the blood-brain barrier, thus making it more permeable.
A critical distinction between D3 and NGF is that D3 binds only to a receptor called TrkA (pronounced “Track A”), which activates a neuroprotective pathway, and not p75, a receptor that can cause cell death and inflammation. In contrast, NGF binds to both receptors. The D3 molecule was developed by Dr. Uri Saragovi at the Lady Davis Institute, Jewish General Hospital, McGill University.
“Dr. Saragovi published several papers on D3 showing it binds only to TrkA, but he had to do invasive intracranial injections to get it into the brain. When he heard about focused ultrasound, he goes, ‘Wow. That’s wonderful. Can you deliver it?’ We knew that it was safe intravenously and it would be even better when it got into the brain. It was one of those situations where we were already in good shape with the background knowledge of this particular molecule” says Aubert.
Using focused ultrasound to deliver D3 is not only safer, it is also more precise, notes Xhima. “Delivery methods for NGF have been done using [brain] injections. The therapeutic [needs to be] delivered to very small structures deep in the brain. With this surgery, it’s difficult to confirm if the targeting is accurate, whereas with MRI-guided focused ultrasound, we can inject an MRI contrast agent and see where the blood-brain barrier opens to deliver the therapeutic more accurately,” she says.
After treatment, they saw a sign of sweet success: “We developed a sonication strategy to ensure that we’re able to open the blood-brain barrier and not cause damage. Afterwards, we looked at the signalling pathways. We see that TrkA is activated, and the neurons are firing and releasing acetylcholine,” says Xhima. The next step is to determine whether the compound improves memory—something the researchers are studying.
For Aubert, the results achieved with the D3 molecule represent an exciting stride forward. She notes that clinical trials testing drugs that clear amyloid and tau have been disappointing, but combining D3 with such a treatment could be an effective one-two punch. “Nothing has really panned out as being curative, to date. Alzheimer’s disease is so complex. Sure, we have to get rid of the pathology—the plaques and tangles—but we have to help the brain function better. That’s where the neurofunctional components come in.”
Xhima received a Frederick Banting and Charles Best Canada Graduate Scholarship Doctoral Award from CIHR.
Original article: Xhima K, Markham-Coultes K, Nedev H, Heinen S, Saragovi HU, Hynynen K, Aubert I. Focused ultrasound delivery of a selective TrkA agonist rescues cholinergic function in a mouse model of Alzheimer’s disease. Sci Adv. 2020 Jan 22;6(4): eaax6646. DOI: 10.1126/sciadv.aax6646
Source of text:
Sunnybrook Research Institute https://sunnybrook.ca/research/media/item.asp?i=2039&f=focused-ultrasound-first
Learn more here:
Behind the research: Study looks at fighting Alzheimer’s disease in a new way





